Atomic Structure
1. Atoms and Molecules
Atom refers to the smallest particle of an element. It is not found in free form. Two or more atoms of an element combine to form a molecule of that element. Molecule shows stability. It can exist independently. For example, O refers to an atom of Oxygen and O2 refers to a molecule of Oxygen. Similarly, H refers to atom of Hydrogen and H2 refers to a molecule of Hydrogen.
2. Particles of Atom
Electron, proton and neutron are constituent particles of an atom. They are also called sub-atomic particles.
2.1 Electron: Electron was discovered by J.J. Thomson and named by Stoney. It has minus one charge and negligible mass. It is found outside the nucleus. The mass of an electron is about 1/2000 times the mass of a hydrogen atom.
2.2 Proton: Proton was discovered by Goldstein and named by Rutherford. It has plus one charge and its mass is taken as one unit. It is found inside the nucleus.
2.3 Neutron: Neutron was discovered by James Chadwick. It has no charge and its mass is equal to proton. It is found inside the nucleus. Neutrons are present in the nucleus of all atoms, except hydrogen.
Almost all the important details about electron, proton and neutron are summarized and provided in the table given below:
|
Detail |
Electron |
Proton |
Neutron |
|
Discovery |
J.J. Thomson |
Goldstein |
James Chadwick |
|
Named by |
Stoney |
Rutherford |
- |
|
Charge/Relative Charge |
Minus one |
Plus one |
No charge |
|
Absolute charge |
-1.6 x 10-19 coulomb |
+1.6 x 10-19 coulomb |
No charge |
|
Mass (Relative Mass) |
Negligible (0.0000543 amu) |
Taken as one unit (1.00763 amu) |
Equal to Proton (1.00863 amu) |
|
Absolute Mass |
9.1 x 10-28 g 9.1 x 10-31 kg |
1.673 x 10-24g 1.673 x 10-27 kg |
1.675 x 10-24g 1.675 x 10-27 kg |
|
Location |
Outside nucleus |
Inside Nucleus |
Inside Nucleus |
|
Name of related experiments/phenomenon |
Cathode rays Experiment |
Anode rays experiment |
Radioactivity phenomenon |
3. Models for Atomic Structure
3.1 Dalton’s atomic theory
In 1808, Dalton presented his atomic theory. It provided an explanation for the laws of chemical combination. It states that all matter, whether an element, a compound or a mixture, is composed of small particles called atoms.
Basic principles of Dalton’s Atomic Theory are:
- All matter is made of very tiny particles called atoms, which participate in chemical reactions.
- Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
- While atoms of a given element are identical in mass and chemical properties, atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
3.2 Model proposed by Thomson
- Thomson’s model for the atomic structure is comparable to Christmas pudding or a watermelon.
- In this model, electrons were like dry fruits in a spherical Christmas pudding or like seeds in watermelon.
- As per this model, an atom is a positively charged sphere with negatively charged electrons embedded in it. Atom is electrically neutral.
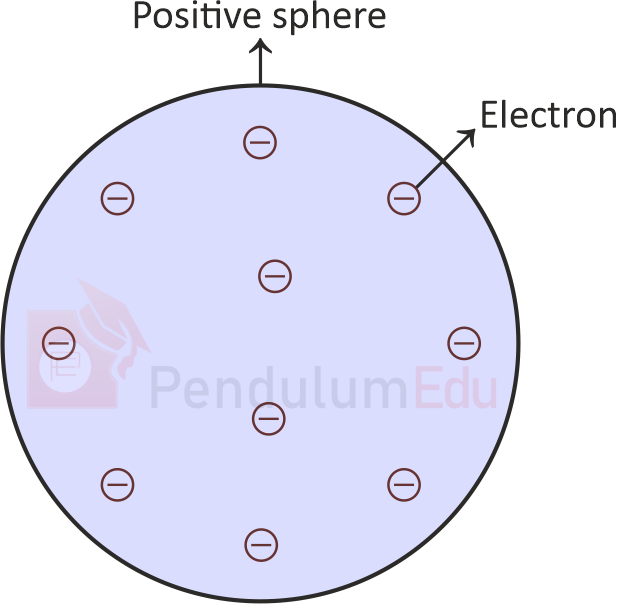
(Fig: Thomson’s Model)
3.3 Model proposed by Rutherford
- It is also called the nuclear model of atom. It was based on Rutherford’s alpha-particle scattering experiment.
- This model proposed positively charged centre in an atom has all the mass of an atom and is called the nucleus. Its size is very small in relation to the atom’s size.
- As per Rutherford’s model, electron revolves around the nucleus in a circular path.
- Drawback of Rutherford’s Model: Rutherford’s atomic model could not explain why revolving electrons do not fall into the nucleus or why atoms are stable.
3.4 Model proposed by Neils Bohr
- According to Bohr’s model, certain special orbits of electrons are found inside the atom.
- These orbits or shells are shown as K, L, M, N,… or as, n=1,2,3,4.
- These shells can have maximum 2, 8, 18 and 32 electrons, respectively.
- Outermost orbit can have 8 electrons only.
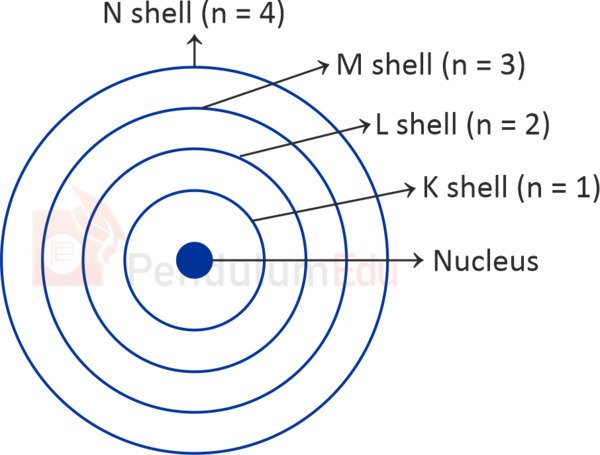
(Fig: Bohr’s Model- Orbits)
4. Terms and Definitions
4.1 Atomic number (Z)
Atomic number (Z) is equal to the number of protons or electrons in an atom. For example, Nitrogen (N) atom has seven protons, seven electrons and seven neutrons. Its atomic number (Z) is seven.
4.2 Mass number (A)
Mass number (A) is equal to the total of protons and neutrons in the nucleus of an atom. Mass number (A) of Nitrogen is fourteen.
4.3 Isobars
Isobars have the same mass number (A). But, they have a different atomic number (Z). Calcium and Argon are examples of isobars having the same mass number (40) and different atomic numbers (20 in case of Calcium and 18 in case of Argon).
4.4 Isotopes
Isotopes have the same atomic numbers (Z). But, they have a different mass number (A). Hydrogen has three isotopes named Protium (A=1), deuterium (A=2) and tritium (A=3). Atomic number (Z) for all of them is 1. Isotope of Uranium is used as fuel in nuclear reactors. Isotope of cobalt and iodine is used in the treatment of cancer and goiter, respectively.
4.5 Isotones
Number of neutrons is the same in isotones. But, they have different atomic numbers (Z). Carbon (Z=8), Nitrogen (Z=8) and Oxygen (Z=8) are isotones. All three have eight neutrons.
4.6 Atomic Mass and Molecular mass
It is found using the mass of Carbon-12 isotope as reference. One-twelfth of the mass of an atom is taken as one atomic mass unit. It was earlier written as amu. It is now written as u. Molecular mass of a substance is equal to the total of atomic masses of all atoms found in its one molecule.
Summary
- Atom refers to the smallest particle of an element.
- Two or more atoms of an element combine to form a molecule of that element.
- Atom consists of three sub-atomic particles. They are electron, proton and neutron.
- Thomson, Rutherford and Neils Bohr have proposed models to explain atomic structure.
- Thomson’s model:
- Comparable to Christmas pudding or a watermelon
- Electrons like dry fruits in a pudding or like seeds in watermelon
- Rutherford’s model:
- Nuclear model of atom and based on Rutherford’s alpha-particle scattering experiment.
- Positively charged centre in an atom called the nucleus. electron revolves around it
- Neils Bohr’s model proposed that certain special orbits of electrons are found inside the atom.
- Atomic number (Z) is equal to the number of proton or electron in an atom.
- Mass number (A) is equal to total of protons and neutrons in the nucleus of an atom.
- Isobars: Same mass number (A), different atomic number (Z). For example, Calcium and Argon.
- Isotopes: Same atomic numbers (Z), different mass number (A) For example, protium , deuterium and tritium.
- Isotones: Number of neutrons same, different atomic numbers (Z). For example, Carbon, Nitrogen and Oxygen.
- One twelfth of the mass of an atom is taken as one atomic mass unit.
- Molecular mass is equal to total of atomic masses of all atoms in one molecule of a substance.
Frequently Asked Questions (FAQs) about Atomic Structure





 Latest
Latest 



Comments