Periodic classification of elements- Law of Triads, Law of Octaves, Mendeleev’s Periodic Table, Modern Periodic Table | Periodic Properties | Types of Elements
The Periodic table has been created to study the chemical properties of elements and to keep a systematic record. In this article, we have discussed doberenier’s triads, law of octaves, Mendeleev's periodic table, modern periodic law, features of periodic table, trends in periodic table, types of elements and periodic table block-s,p,d,f elements.
1. Periodic Table Genesis
1.1. Dobereiner’s Triads
In 1829, J.W. Dobereiner formed groups of three elements and called them triads. All three elements of a triad were similar in their physical and chemical properties. According to Dobereiner’s law of triads, when elements are arranged in order of increasing atomic mass, the atomic mass of the middle element was nearly equal to the arithmetic mean of the other two and its properties were intermediate between those of the other two.
For example: Lithium, Sodium and Potassium having atomic mass 7, 23 and 39 respectively can be placed together as the atomic mass of sodium is equal to the arithmetic mean of Lithium and Potassium.
1.2. Newlands’ Law of Octaves
John Alexander Newlands in 1864 developed a system of classification of elements and termed it as Law of Octaves. He arranged the elements in increasing order of their atomic masses and observed that every eighth element had properties same as that of the first element.
| Arrangement of some elements as per Law of Octaves | ||||||
| Li (7) | Be (9) | B (11) | C (12) | N (14) | O (16) | F (19) |
| Na (23) | Mg (24) | Al (27) | Si (28) | P (31) | S (32) | Cl (35.5) |
| K (39) | Ca (40) |
|
|
|
|
|
Merits of Law of Octaves: Atomic mass was the basis of classification and periodicity of properties was recognized.
Demerits of Law of Octaves: It was not applicable to the elements with an atomic mass more than 40. The discovery of noble gases made it clear that it was not the eighth element that had similar properties but the ninth one.
1.3. Mendeleev’s Periodic Table
Mendeleev, a Russian Chemist, had studied the relation between the atomic weights of the elements and their physical and chemical properties in 1869.
He constructed a table in which elements were arranged in order of their increasing atomic weights. He is also known as the Father of Modern Periodic Table.
According to Mendeleev’s Periodic law, the chemical and physical properties of elements are a periodic function of their atomic masses.
He corrected the atomic masses of certain elements. Although, he did leave gap under some elements like Aluminium as he believed that some elements were still undiscovered. Mendeleev’s periodic table didn’t give place to isotopes and noble gases.
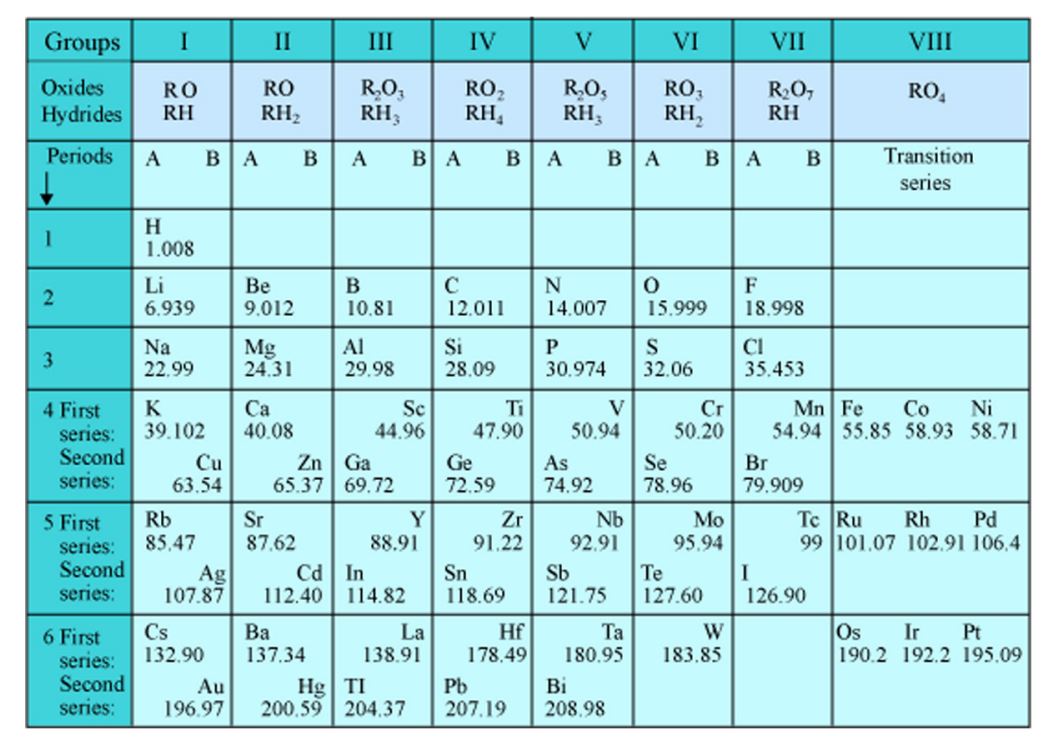
Fig.1: Mendeleev’s Updated Periodic Table (Source: NIOS)
1.4. Modern Periodic Table
The atomic number was discovered by Henry Moseley in 1913. He arranged elements according to increasing atomic numbers.
Moseley proposed that “Properties of the elements are the periodic function of their atomic numbers”.
Atomic Number (Z) is defined as the number of protons present in the nucleus of the atom of an element.
Modern Periodic Law - The chemical and physical properties of elements are periodic functions of their atomic numbers i.e. if elements are arranged in the order of their increasing atomic number, the elements with similar properties are repeated after certain regular intervals.
2. Properties of Modern Periodic Table
- The elements are arranged in rows and columns in the periodic table.
- It has 18 vertical columns called GROUPS. Every group has a unique configuration. There are eighteen groups numbered from I to VIII (Roman numerals).
- It has seven horizontal rows called “periods”. The periods have been numbered from 1 to 7.
- Groups I to VII are further divided into A and B subgroups.
- All the elements present in a particular group are chemically similar in nature.
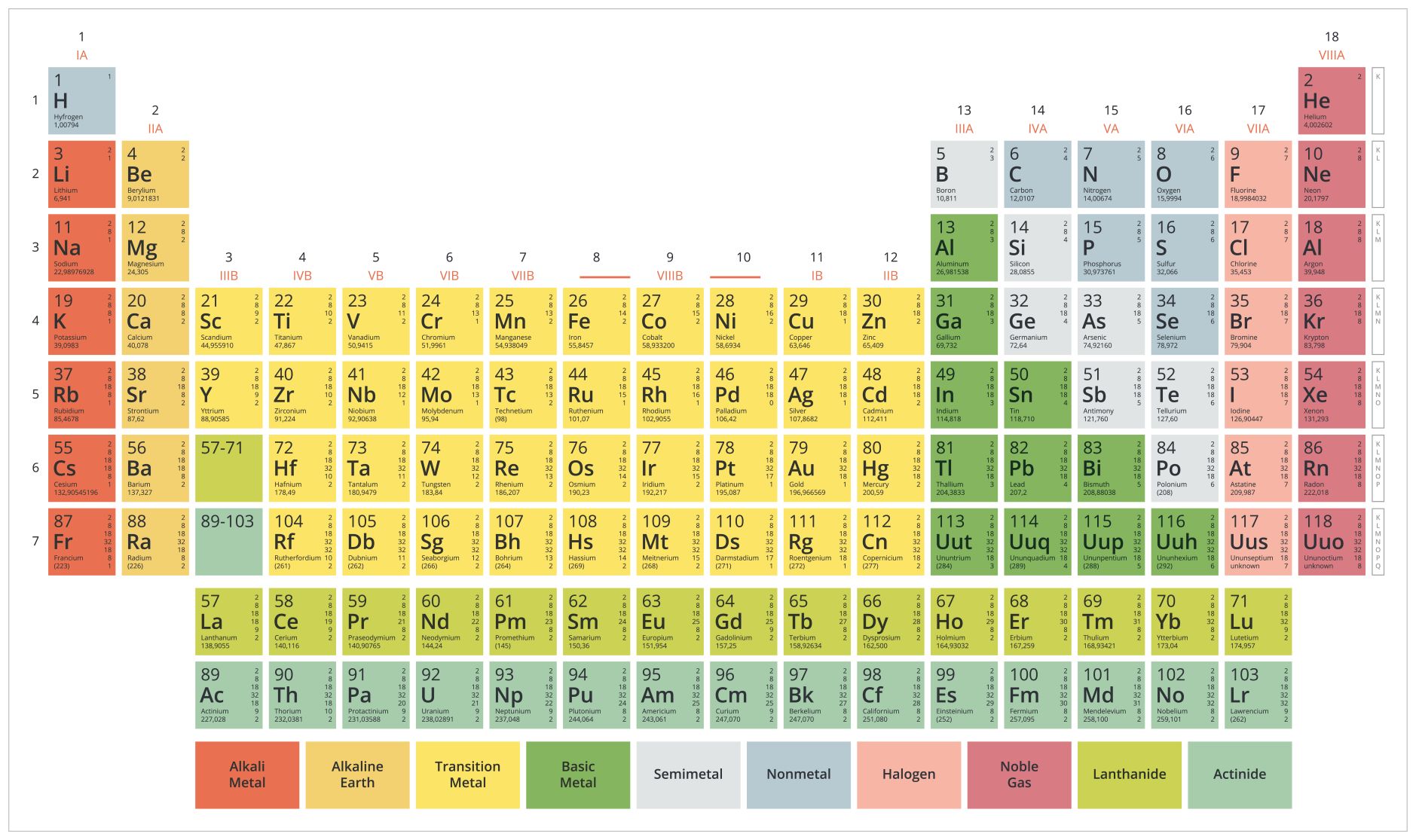
Fig.2: Modern Periodic Table
2.1. Atomic Size
- The term atomic size refers to the radius of an atom. It increases as the number of shells increases.
- It is measured in picometer. The size of atoms decreases from left to right in a period but increases from top to bottom in a group.
- Alkali Metals have the largest atomic size while halogens have the smallest atomic size.
2.2. Valency
- The valency of an element is determined by the number of valence electrons present in the outermost shell of an atom.
- It increases from 1 to 7 along a period with respect to oxygen. It first increases from 1 to 4 and then decreases to 0 with respect to hydrogen.
2.3. Metallic and non-metallic properties
- The tendency of an element to lose electrons to form cations is called the electropositive or metallic character of an element.
- The tendency of an element to accept electrons to form anions is called the electronegative or non-metallic character of an element.
- Metallic character increases from top to bottom in a group and decreases in a period from left to right.
| Property | In a Period (From left to right) | In a Group (From top to bottom) |
| Atomic number | increases | increases |
| Atomic size | decreases | increases |
| Metallic character | decreases | increases |
| Non-metallic character | increases | decreases |
2.4. Electronegativity
- Electronegativity is defined as a measure of the ability of an atom to attract the electron pair in a covalent bond to itself.
- The value of electronegativity decreases on going down in any group and increases from left to right in the period.
- Fluorine is the most electronegative and Cesium is the least electronegative element.
2.5. Ionization Enthalpy
- Ionization Enthalpy is the energy required to remove free electron from an isolated atom in the gaseous state for one mole of an element. It is expressed in kJ mol–1 (kilojoules per mole).
- It increases as we move from left to right in the periodic table.
- The variation in Ionization Enthalpy depends upon the size of the atom, magnitude of the nuclear charge on atom and the types of orbital.
3. Periodic Table blocks
3.1. s-block elements
- These are the elements whose valence electrons enter into s-orbital.
- Group-1 (alkali metals) and Group-2 (alkali earth metals) are part of the s-block in the periodic table.
- These elements are soft metals and form basic oxides.
- The general configuration of s block elements is ns1 for alkali metals and ns2 for alkaline earth metals.
- These elements are never found pure in nature due to high reactivity.
3.2. p-block elements
- The general configuration of p- block elements is ns2np1 to 6.
- Group 13-18 are part of the p-block elements in the periodic table.
- It is the only block that contains metals, non-metals, and metalloids.
- p-block and s-block elements are together called the Representative Elements or Main Group Elements.
- Group 16 and Group 17 elements are called Chalcogens and Halogens respectively.
- The non-metallic character increases on moving from left to right across a period.
3.3. d-block elements
- The elements in this block are called transition elements. Zinc, cadmium and mercury are excluded from category of transition elements.
- The valence electron of elements in this block enters in d-orbital.
- The general configuration of d-block elements is (n-1) d1-10ns1-2.
- Elements from Group 3-12 are part of this block. They are all metals.
- These are often used as catalysts.
3.4. f-block elements
- f-block elements are arranged in two series- lanthanoids and actinoids.
- The general Electronic configuration of lanthanoids is 4f1–14 5d0–1 6s2.
- The general Electronic configuration of actinoids is 5f1–14 6d0–1 7s2.
- Elements of f-block are also known as Inner-transition elements.
- Actinoid elements are radioactive.
- Elements after Uranium are known as Transuranium Elements.
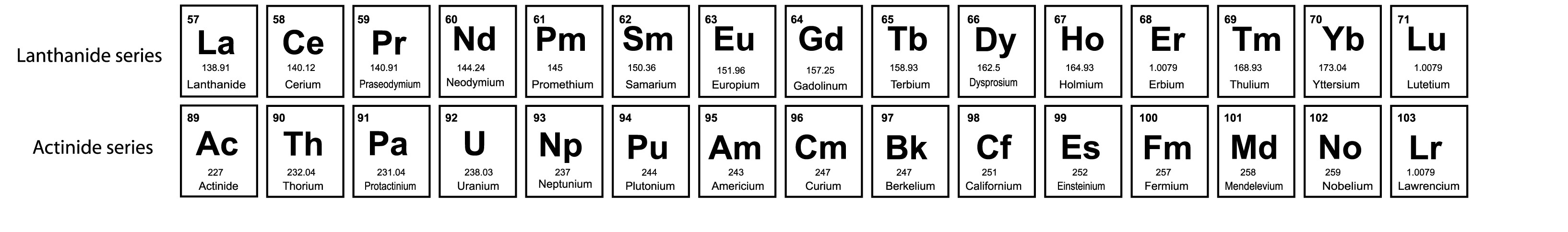
Fig.3: f-block elements
4. Characteristics of Periods in periodic table
- The periods have been numbered from 1 to 7 and elements in a period have consecutive atomic numbers.
- The first period is the shortest period of all. There are only two elements- H and He.
- The second and third periods are short periods with 8 elements each.
- The fourth and fifth periods are called long periods with 18 elements each.
- The sixth and seventh periods are very long periods with 32 elements each.
- The valency of elements increases from 1 to 4 and then decreased to 0 on moving left to right in a period.
- Atomic size, electropositive nature, metallic character, reducing nature of elements decrease from left to right.
- Electronegativity, electron affinity, and non-metallic nature increase from left to right.
5. Characteristics of Groups in periodic table
- The groups have been numbered from 1 to 18 (in Arabic numerals).
- All elements present in a group have similar electronic configurations and same valence electrons.
- Atomic size, electropositive nature, metallic character, reducing nature of elements increases from top to bottom.
- Electronegativity, electron affinity, and non-metallic nature decrease down the group.
Note:
Group 1 elements except hydrogen, are called Alkali Metals.
Group 2 elements are called Alkaline Earth Metals.
Group 3 to 12 elements are called Transition Metals.
Group 16 elements are called Chalcogens.
Group 17 elements are called Halogens.
Group 18 elements are called Noble Gases.
6. Types of Elements
6.1. Metals and Non-Metals
Metals are present on the left-hand side of the periodic table while Non-metals occupy the right- hand portion of the periodic table.
Alkali metal and alkaline earth metals occupy groups 1 and 2 respectively.
Halogens and chalcogens elements occupy group 17 and 16 respectively.
6.2. Metalloids
Elements that show mixed properties of metal and non-metal are known as Metalloids. They are present along the diagonal line starting from group 13 (Boron) and going down to group 16 (Polonium).
6.3. Noble Gases
Group 18 contains noble gases and they are located on the extreme right of the periodic table. Their outermost shells contain 8 electrons and have zero valency. They are non-reactive and all of them are gases.
6.4. Transition Elements
Groups 3 to 12 contain the Transition Elements and their outermost shells are incomplete. They are good conductors of heat and electricity and most of them are used as catalyst. They have high melting and boiling points.
6.5. Inner Transition Elements
They are also known as rare-earth elements. These are two series of 14 elements each. The first series is called lanthanoids while the second series of 14 elements is known as actinoids.
Summary
- A Periodic table has been created to study and chemical properties of elements and to keep a systematic record.
- J.A.R. Newlands developed a system of classification of elements and termed it as Law of Octaves.
- Mendeleev studied the relation between the atomic weights of the elements and their physical and chemical properties in 1869.
- The atomic number was discovered by Moseley in 1913. The modern periodic table is based on the atomic number. Henry Moseley arranged elements according to increasing atomic numbers.
- Modern periodic table has 18 vertical columns called GROUPS and seven horizontal rows called PERIODS.
- The term atomic size refers to the radius of an atom. It increases as the number of shells increases.
- The valency of an element is determined by the number of valence electrons present in the outermost shell of an atom.
- The tendency of an element to lose electrons to form cations is called the electropositive or metallic character of an element.
- The tendency of an element to accept electrons to form anions is called the electronegative or non-metallic character of an element.
- The value of electronegativity decreases on going down in any group and increases from left to right in the period.
- Ionization Enthalpy is the energy required to remove free electron from an isolated atom in the gaseous state for one mole of an element. It is expressed in kJ mol–1 (kilojoules per mole).
- Group-1 and Group-2 are part of the s-block in the periodic table. The general configuration of s block elements is ns1 for alkali metals and ns2 for alkaline earth metals.
- The general configuration of o- block elements is ns2np1-6. It is the only block that contains metals, non-metals, and metalloids.
- The elements in d- block are called transition elements. Elements from Group 3-12 are part of this block.
- f- Block consists of two series of elements called lanthanoids and actinoids. Elements of f-block are also known as Inner-transition elements.
- Atomic size, electropositive nature, metallic character, reducing nature of elements decrease from left to right.
- Atomic size, electropositive nature, metallic character, reducing nature of elements increase from top to bottom.
- Elements that show mixed properties of metal and non-metal are known as Metalloids.
- Group 18 contains noble gases and they are located on the extreme right of the periodic table.




 Latest
Latest 



Comments