Combustion and Flame Class 8 Notes NCERT and MCQs
 23-08-2023
23-08-2023
 17:13 PM IST
17:13 PM IST
 Yadvendra Singh
Yadvendra Singh
The chapter discusses combustion, spontaneous combustion, ignition temperature, inflammable substances and essential requirements of fire and its control. It also describes characteristics of good fuel and harmful effects of burning of fuel on the environment.
What is Combustion?
A chemical process in which a substance reacts with oxygen to give off heat is called combustion. Air is necessary for combustion. The substance that undergoes combustion is said to be combustible. It is also called fuel. Substances that do not catch fire easily are non-combustible substances. For example- Sand, Water, etc.
Spontaneous combustion
The type of combustion in which a material suddenly bursts into flames, without the application of any apparent cause is called spontaneous combustion. Spontaneous combustion of coal dust is the cause of many disastrous fires in coal mines.
Ignition temperature
The lowest temperature at which a substance catches fire is called its ignition temperature. It is essential for a substance to reach ignition temperature to burn. A combustible substance cannot catch fire or burn as long as its temperature is lower than its ignition temperature.
Inflammable substances
They are substances that have very low ignition temperature and can easily catch fire with a flame. Petrol, alcohol and Liquified Petroleum Gas (LPG) are some examples of inflammable substances.
Fire and its control
Three essential requirements for producing fire are fuel, air (to supply oxygen) and heat. Heat is required to raise the temperature of the fuel beyond the ignition temperature. Fire can be controlled and extinguished by cutting off the supply of air, or by bringing down the temperature of the fuel below its ignition temperature, or both.
Fire extinguishers: The most common fire extinguisher is water. But it is not suitable for fires involving electrical equipment and inflammable materials like petrol. In such cases, carbon dioxide (CO2) is the best extinguisher. It is heavier than oxygen and covers the fire like a blanket to cut off the contact between the fuel and oxygen. It also brings down the temperature of the fuel as it cools down when released from the cylinder.
Flame
Sometimes, light is also given off during combustion, either as a flame or as a glow. Substances such as kerosene oil and molten wax which vapourise during burning, give flames. Charcoal does not vapourise and so does not produce a flame.
Structure of flame
Unburnt carbon particles are present in the luminous zone of the flame. The outermost part is the non-luminous zone of the flame and is the hottest part. It is blue in color.
The middle zone is a zone of partial combustion and is yellow in color. It is the brightest part and moderately hot.
The innermost zone is the least hot area. It is black in color as unburnt wax vapors are present here.

Fig.1: Different zones of candle flame
Fuel
Substances that are sources of heat energy for domestic and industrial purposes are called fuels.
Characteristics of a good fuel
- It is readily available.
- It is cheap.
- It burns easily in air at a moderate rate.
- It produces a large amount of heat.
- It does not leave behind any undesirable substances.
Fuel efficiency/ calorific value
The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its calorific value. Its unit is kilojoule per kg (kJ/kg). Fuels like CNG, LPG, Biogas, Hydrogen have high calorific value while Wood has low calorific value.
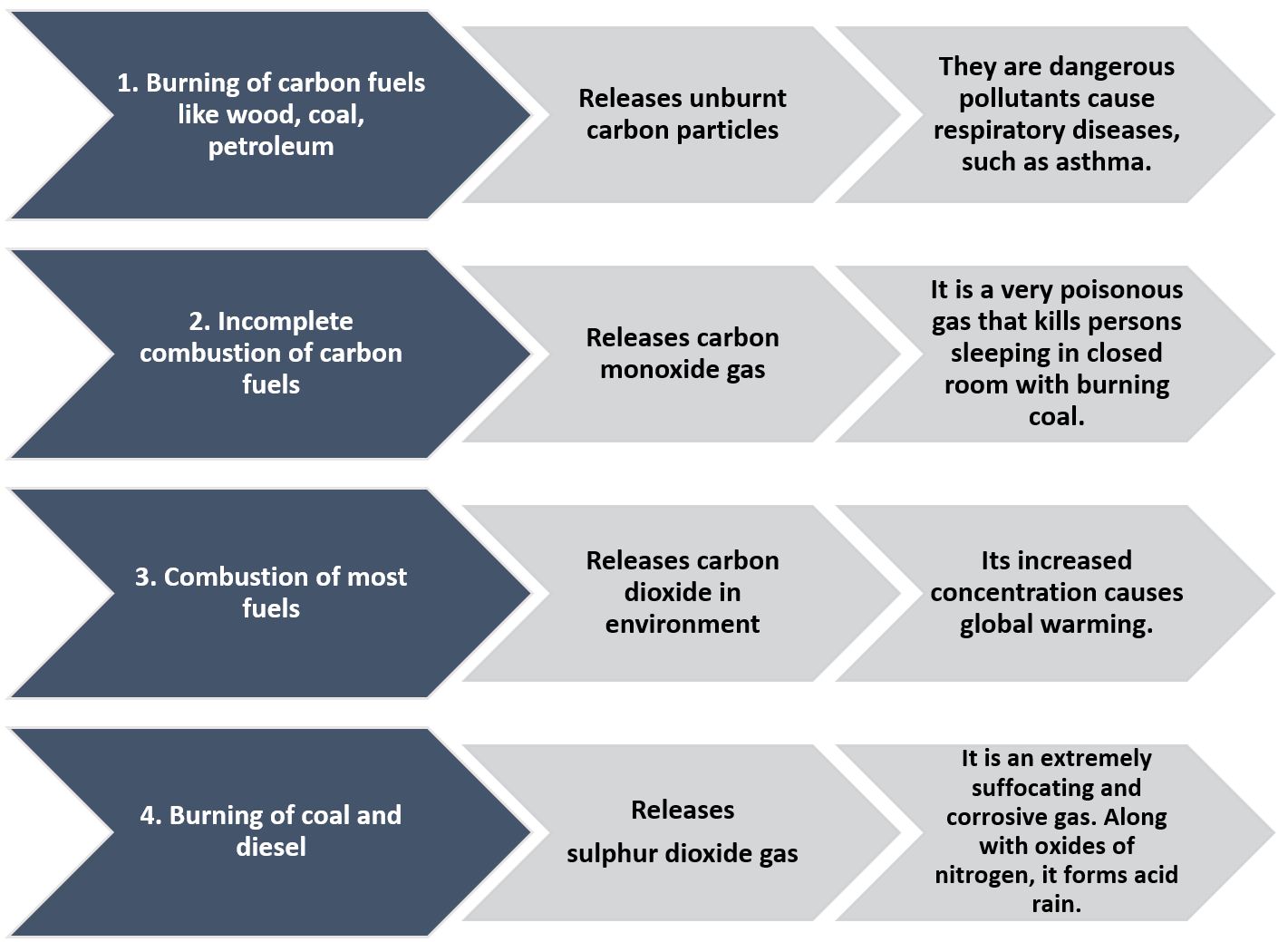
Harmful Impacts of Burning of Fuels
1. Burning of carbon fuels like wood, coal, petroleum releases unburnt carbon particles. They are dangerous pollutants that cause respiratory diseases, such as asthma. Cutting of trees leads to deforestation.
2. Incomplete combustion of carbon fuels releases carbon monoxide gas. It is a very poisonous gas that kills persons sleeping in closed room with burning coal.
3. Combustion of most fuels releases carbon dioxide in environment. Its increased concentration causes global warming.
4. Burning of coal and diesel releases sulphur dioxide gas. It is an extremely suffocating and corrosive gas. Along with oxides of nitrogen, it forms acid rain.
Therefore, the use of diesel and petrol as fuels in automobiles is being replaced by CNG as it is a cleaner fuel. Also, the use of coal, wood, etc, as a domestic fuel has been replaced by fuels like LPG.
Fig.2: Harmful effects of burning of fuels
MCQs based on NCERT Class 8 Chapter 6: Combustion and Flame
1. The lowest temperature at which a substance catches fire is called-
a. Ignition temperature
b. Combustion temperature
c. Calorific value
d. Flame temperature
Ans. a
Explanation:
The lowest temperature at which a substance catches fire is called its ignition temperature.
2. Which of the following is suitable to extinguish fires involving inflammable materials like petrol?
a. Water
b. Carbon dioxide
c. Nitrogen dioxide
d. Hydrogen
Ans. b
Explanation:
The most common fire extinguisher is water. But it is not suitable for fires involving electrical equipment and inflammable materials like petrol. In such cases, carbon dioxide (CO2) is the best extinguisher. It is heavier than oxygen and covers the fire like a blanket to cut off the contact between the fuel and oxygen. It also brings down the temperature of the fuel as it cools down when released from cylinder.
3. Which zone of a flame does a goldsmith use for melting gold and silver?
a. Blue Zone
b. Yellow Zone
c. Black Zone
d. Green Zone
Ans. a
Explanation:
Goldsmiths use the outermost zone of the flame i.e. Blue zone for melting gold and silver. It is the zone of complete combustion.
Frequently Asked Questions (FAQs) about Combustion and Flame
Name the unit in which the calorific value of a fuel is expressed.
Why water is not used to control fires involving electrical equipment?
Why is it difficult to burn a heap of green leaves but dry leaves catch fire easily?
What do the fuels produce when they burn?
Share Blog
 Latest
Latest 
Comments