Matter in Our Surroundings Class 9 Notes and MCQs
 14-03-2024
14-03-2024
 16:03 PM IST
16:03 PM IST
 Priyanka Chaudhary
Priyanka Chaudhary
Everything around us, it is either the air we breathe, food, clouds, stars or sand particles, is made up of material called “matter”. Here, we will discuss what is matter and its physical properties.
What is matter?
Anything that occupies space and has mass is called matter. In other words, matter has both volume and mass.
Early Indian philosophers classified matter in the form of five basic elements — the “Panch Tatva” — air, earth, fire, sky and water and everything, living or non-living, was made up of these five basic elements.
Modern day scientists have evolved two types of classification of matter based on their physical properties and chemical nature.
Physical Nature of Matter
If we dissolve some sugar or salt in 50 mL of water its level will not change as the particles get into the space between the particles of water. Hence, we can say that Matter is made up of particles.
There are a million of tiny particles present in matter which keep on dividing themselves into smaller and smaller particles as evident from the given below experiment:
1. Take 2–3 crystals of potassium permanganate and dissolve them in 100 mL of water.
2. Take out approximately 10 mL of this solution and put it into 90 mL of clear water.
3. Take out 10 mL of this solution and put it into another 90 mL of clear water.
4. Keep diluting the solution like this 5 to 8 times.
What do you observe? Is water still coloured?- Although the color becomes light with every dilution, yet it is still visible.
Characteristics of Particles of Matter
1. Particles of Matter have space between them: Whenever we make tea, coffee, or lemonade, particles of one type gets evenly distributed and get into the space present between the particles of the other type. This shows that there is enough space between particles of matter.
2. Particles of Matter are continuously moving: The particles of matter possess kinetic energy. As the temperature increases, the particles move faster. Hence, it can be inferred that with the increase in temperature the kinetic energy of the particles also increases.
Diffusion: The intermixing of particles of two different types of matter on their own is called diffusion. On heating, the process of diffusion becomes faster.
Experimental observations:
a. When we lit the incense stick we can smell it from a distant place as its fragrance spreads all around due to the diffusion of its smoke in the air.
b. When a drop of colored ink and honey is put slowly along the sides of two different beakers filled with water and they are left undisturbed. After sometime, you will observe that the ink drop will spread faster as compared to honey as the density of honey is more than that of ink, hence, the particles of ink diffuses faster as compared to honey particles.
c. A crystal of copper sulphate or potassium permanganate when dropped into a glass of hot water and another in cold water without stirring. Allow the crystals to settle in bottom. You will observe that in a glass of cold water, the crystals settle down to the bottom and in case of hot water, it slowly spreads. The crystals can be observed dissolving in the water just above and blue color is observed. After some time, the hot water becomes blue and no major color change is observed in cold water because of less dissolution of crystals. The above observation shows that particles of matter move continuously and possess kinetic energy. The particles of matter also have diffusion property. As the kinetic energy of particles increases with the increase of temperature, therefore, the rate of mixing also increases as observed in the case of hot and cold water.
3. Particles of Matter Attract Each other: Particles of matter have force acting between them and this force keeps the particles together. The strength of the force of attraction varies from one kind og matter to another.
Experimental Observations:
a. When we take an iron nail, a piece of chalk and a rubber band and try breaking them by hammering, cutting or stretching. You will observe that in case of iron nail the particles are held together with greater force.
b. If we take some water in a container and try cutting the surface of water with your fingers. You will observe that you are unable to cut the surface of water as particles of water are held together by greater force.
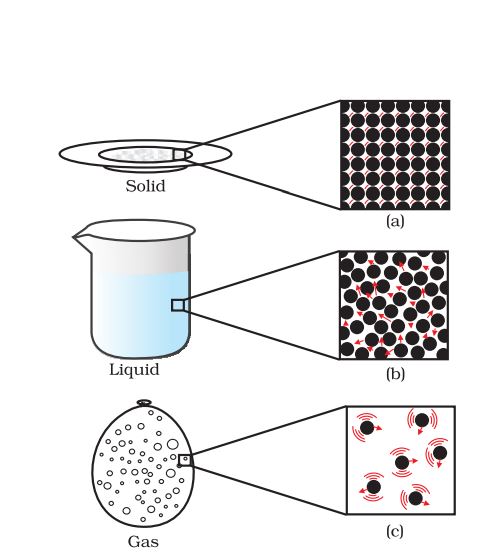
States of Matter
Matter around us exists in three different states– solid, liquid and gas. These states of matter arise due to the variation in the characteristics of the particles of matter.
THE SOLID STATE
Pen, Book, Needle, Piece of wooden stick are all examples of solids. The other examples of solids are:
- A rubber band changes shape under force and regains the same shape when the force is removed. If excessive force is applied, it breaks.
- The shape of each individual sugar or salt crystal remains fixed, whether we take it in our hand, put it in a plate or in a jar.
- A sponge has minute holes, in which air is trapped, when we press it, the air is expelled out and we are able to compress it.
Properties of solids are as follows:
1. They have a definite shape, distinct boundaries and fixed volumes.
2. They have negligible compressibility.
3. Solids have a tendency to maintain their shape when subjected to outside force. Solids may break under force but it is difficult to change their shape, so they are rigid.
THE LIQUID STATE
Water, cooking oil, milk, juice, cold drink are all examples of liquids.
Properties of liquids are as follows:
1. Liquids have no fixed shape but have a fixed volume.
2. They take up the shape of the container in which they are kept.
3. Liquids flow and change shape, so they are not rigid but can be called fluid.
4. Solids, Liquids and Gases can diffuse into Liquids. The gases from the atmosphere diffuse and dissolve in water. The aquatic animals can breathe under water due to the presence of dissolved oxygen in water.
NOTE: The rate of diffusion in liquids is higher than that of solids because in liquid state, particles move freely and have greater space between each other as compared to particles in the solid state.
THE GASEOUS STATE
Air is a mixture of gases like oxygen, nitrogen, carbon dioxide, etc.
Properties of Gases are as follows:
1. Gases are highly compressible as compared to solids and liquids. Ex- CNG used in vehicles and LPG used in our homes are compressed into a small cylinder and transported easily.
2. Gases show the property of diffusing very fast into other gases, due to high speed of particles and large space between them.
3. In the gaseous state, the particles move about randomly at high speed. Due to this random movement, the particles hit each other and also the walls of the container. The pressure exerted by the gas is because of this force exerted by gas particles per unit area on the walls of the container.
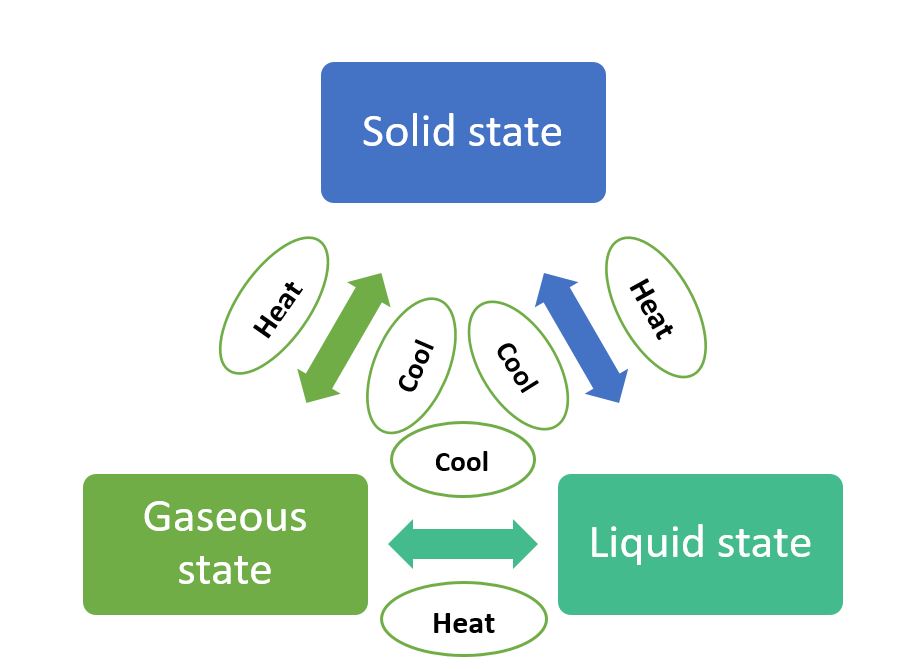
Change in State of Matter
Water can exist in three states of matter:
- solid, as ice
- liquid, as the familiar water, and
- gas, as water vapour
Pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
A. Effect of Change of Temperature
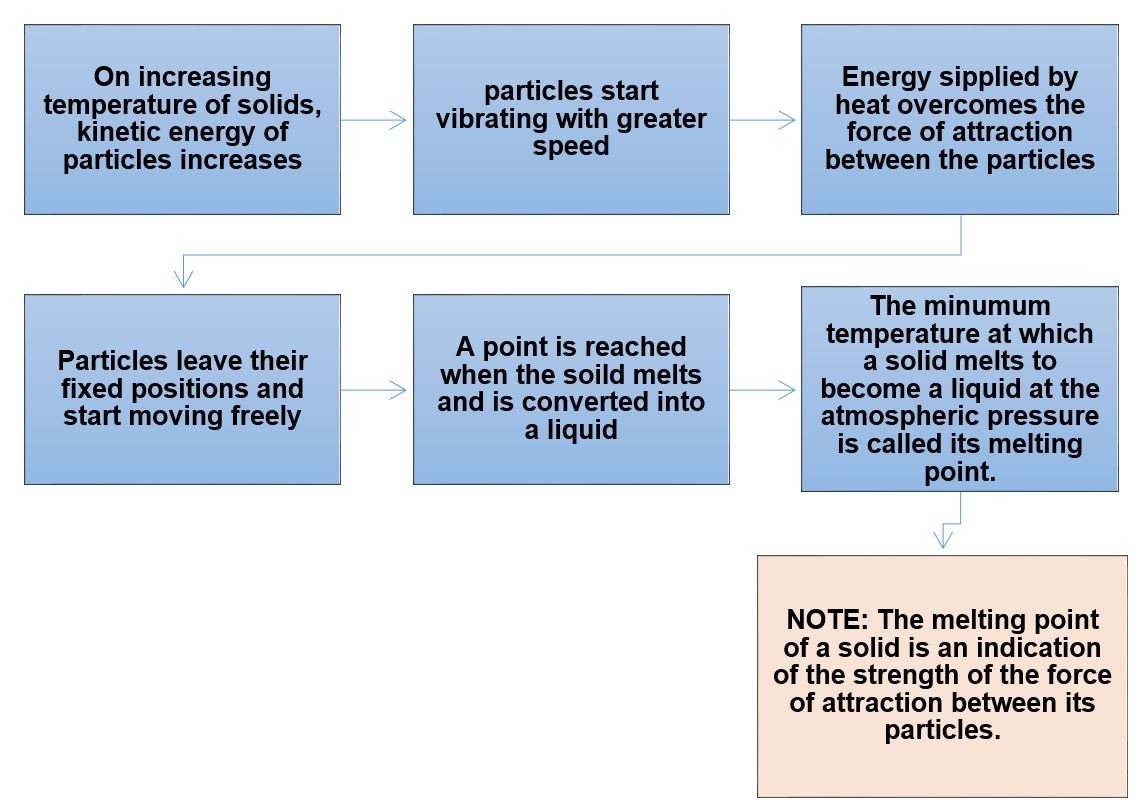
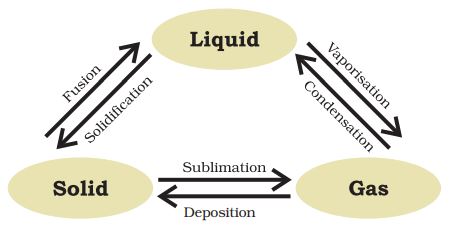
Fusion: The process of melting or change of solid state into liquid state.
The heat absorbed by the ice is used in changing the state by overcoming the forces of attraction between the particles. As the heat energy is absorbed by ice without showing any rise in temperature, it is considered that it gets hidden into the contents of the beaker and is known as the latent heat.
Latent Heat of Fusion: The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
Therefore, the particles in water at 0°C (273 K) have more energy as compared to particles in ice at the same temperature.
Boiling point: The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
Latent Heat of Vapourisation: The amount of heat energy that is required to change 1 kg of a liquid into gas at atmospheric pressure at its boiling point is known as latent heat of vapouriation.
Therefore, particles in steam, that is, water vapour at 373 K (100° C) have more energy than water at the same temperature. This is because particles in steam have absorbed extra energy in the form of latent heat of vaporisation.
Sublimation: A change of state directly from solid to gas without changing into liquid state is called sublimation. Example- Sublimation of camphor
Deposition: The direct change of gas to solid without changing into liquid is called deposition.
B. Effect of Change of Pressure
Particles of matter can be brought together by applying pressure. Example- LPG, CNG, LNG
Solid Carbon dioxide is known as dry ice because it is stored under high pressure and as soon as the pressure is decreased to 1 atmosphere it directly gets converted into gaseous state.
EVAPORATION
The phenomenon of change of liquid into vapours at any temperature below its boiling point is called evaporation.
Particles of matter are always moving. At a given temperature in any gas, liquid or solid, there are particles with different amounts of kinetic energy. In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour.
Factors affecting evaporation
1. Temperature- With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
2. Surface area- evaporation is a surface phenomenon. If the surface area is increased, the rate of evaporation increases. For example, while putting clothes for drying up we spread them out.
3. Wind velocity or Wind speed- With the increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surrounding.
4. Humidity- Humidity is the amount of water vapour present in air. If the amount of water in air is already high, the rate of evaporation decreases.
HOW DOES EVAPORATION CAUSE COOLING?
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings make the surroundings cold.
Need to Know Facts
- SI Unit of mass is kilogram (kg)
- SI unit of volume is cubic metre (m3).
- Common unit of measuring volume is litre (L) such that 1L = 1 dm3 , 1L = 1000 mL, 1 mL = 1 cm3 .
- The mass per unit volume of a substance is called density. (density = mass/volume)
- The melting point of ice is 273.15 K, where Kelvin is the SI unit of temperature and 0°C = 273.15 K.
- A gas fills completely the vessel in which it is kept because there is negligible force of attraction between the particles of gas and they move freely in all directions.
- We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert. In air, the particles are held by the weak force of attraction whereas in the block of wood the particles are closely packed by the strong force of attraction which requires a huge force to overcome the attraction.
- Liquids generally have lower density as compared to solids but ice floats on water because the density of water is maximum at 4°C and the density of ice is less than that of water because of its weak intermolecular force of attraction.
MCQs related to NCERT Class 9 Science Chapter 1: Matter in Our Surrounding
1. What is the SI unit of Weight?
a. Kilogram
b. Kelvin
c. Gram
d. Newton
Ans. d
Explanation:
| Quantity | Unit | Symbol |
| Temperature | Kelvin | K |
| Length | Metre | m |
| Mass | Kilogram | kg |
| Weight | Newton | N |
| Volume | Cubic metre | m3 |
| Density | Kilogram per cubic metre | kg/m3 |
| Pressure | Pascal | Pa |
2. What is the physical state of water at 25°C?
a. Solid
b. Liquid
c. Gas
d. None
Ans. b
Explanation:
At 0°C, water exists in the form of ice and below 100°C it exists in the form of liquid. Above 100°C, water exists in gaseous state.
3. Which of the following produces more severe burns?
a. Water at 70°C
b. Boiling water
c. Steam
d. Water at 50°C
Ans. c
Explanation:
Steam produces more severe burns because the particles of steam contain more energy (latent heat of vapourisation) than the particles of boiling water.
4. Why do we see water droplets on the outer surface of a glass containing ice-cold water?
a. Water vapour present in the air, on coming in contact with cold glass of water, gets converted to water droplets.
b. Moisture present in the air gets stick to the cold glass of water.
c. Water in the glass evaporates and then cool down and gets converted in water droplets.
d. None of the above
Ans. b
Explanation:
We see water droplets on the outer surface of a glass containing ice-cold water because the water vapour present in air, on coming in contact with the cold glass of water, loses energy and gets converted to liquid state, which we see as water droplets.
5. Why should we wear cotton clothes in summer?
a. They look good.
b. They acts as good absorber of sweat.
c. Cotton is a natural fibre.
d. None of the above
Ans. b
Explanation:
During summer, we perspire more because of the mechanism of our body which keeps us cool. We know that during evaporation, the particles at the surface of the liquid gain energy from the surroundings or body surface and change into vapour. The heat energy equal to the latent heat of vaporisation is absorbed from the body leaving the body cool. Cotton, being a good absorber of water helps in absorbing the sweat and exposing it to the atmosphere for easy evaporation.
Frequently Asked Questions (FAQs) about NCERT Class 9 Science Chapter 1 Matter in Our Surrounding
Why does the process of diffusion become faster on heating?
For any substance, why does the temperature remain constant during the change of state?
A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
What are the characteristics of the particles of matter?
Why do feel cool when you pour some nail polish remover (acetone) on your palm?
Why do people sprinkle water on the roof or ground after a hot sunny day?
Share Blog
 Latest
Latest 
Comments