Reduction and Oxidation
The concept of Reduction and Oxidation is very important for the students preparing for competitive exams like JEE, NEET, RRB NTPC, SSC CGL, SSC CHSL, CDS, NDA, etc . Here we have discussed about the important terms like reduction, oxidation, redox reaction, and the ways to calculate oxidation number.
1. Classical concept of oxidation and reduction
What is Oxidation?
Oxidation is a reaction in which there is addition of oxygen or removal of hydrogen. It is also defined as a reaction in which an electronegative element or group is added or an electropositive element or group is removed.
|
Examples of Oxidation |
|
|
Oxidation of Mg |
2Mg + O2 → 2MgO |
|
Oxidation of H2S |
H2S + Cl2 → 2HCl + S |
|
Oxidation of Fe |
Fe + S → FeS |
|
Oxidation of KI |
2KI + H2O2 → 2KOH + I2 |
What is Reduction?
Reduction is a reaction in which there is addition of hydrogen or removal of oxygen. It is also defined as a reaction in which an electropositive element or group is added or an electronegative element or group is removed.
| Examples of Reduction | |
|
Reduction of Cl2 |
H2 + Cl2→ 2HCl |
|
Reduction of CuO |
CuO + C → Cu + CO |
|
Reduction of HgCl2 |
HgCl2 + Hg → Hg2Cl2 |
|
Reduction of FeCl3 |
2FeCl3 + H2 → 2FeCl2 + 2HCl |
1.1. Oxidation Vs Reduction
|
|
Oxidation |
Reduction |
|
Addition of |
Oxygen |
Hydrogen |
|
Electronegative element/group |
Electropositive element/group |
|
|
Removal of |
Hydrogen |
Oxygen |
|
Electropositive element/group |
Electronegative element/group |
2. Modern concept of oxidation and reduction
According to modern concept, oxidation is defined as loss of electrons and reduction is defined as gain of electrons.
|
Oxidation and Reduction (Modern Concept) |
|
|
Na → Na+ + e (Oxidation of Na) |
Cl2 + 2e → 2Cl- (Reduction of Cl2) |
|
Zn → Zn2+ + 2e (Oxidation of Zn) |
S + 2e → S2- (Reduction of S) |
3. Effects of Oxidation in Everyday Life
Corrosion and rancidity are effects of oxidation reaction in everyday life. Rusting of iron, black coating on silver and the green coating on copper are examples of corrosion. Fats and oils become rancid and their smell and taste change when they are oxidized. Antioxidants are substances that prevent oxidation. They are added to foods having fats and oils. Foods are kept in air tight containers to slow down oxidation.
4. What is an Oxidizing agent?
Oxidizing agent (oxidant) is a substance that undergoes reduction and that is acceptor of electrons. It is the substance which gets reduced. Its oxidation number is reduced. Oxygen (O2), ozone (O3), hydrogen peroxide (H2O2), potassium permanganate (KMnO4), potassium dichromate (K2Cr2O7) are some examples of oxidizing agents.
1. CuO + C → Cu + CO (In this reaction, CuO undergoes reduction. Hence, it is an oxidizing agent.)
2. Zn(s) + 2HCl (aq) → ZnCl2 (aq) + H2 (In this reaction, there is a reduction in oxidation number of H from +1 to 0. H is reduced in this reaction. So, H in HCl is the oxidizing agent.)
5. What is a Reducing agent?
Reducing agent (reductant) is a substance that undergoes oxidation and that is donor of electrons. It is substance which gets oxidized. Its oxidation number is increased. Hydrogen (H2), sulphur dioxide (SO2), carbon monoxide (CO), hydrogen sulphide (H2S) and carbon (C) are examples of reducing agents.
1. H2O + C → CO + H2 (In this reaction, C undergoes oxidation. Hence, it is a reducing agent.)
2. Zn(s) + 2HCl (aq) → ZnCl2 (aq) + H2 (In this reaction, there is an increase in oxidation number of Zn from 0 to +2. Zinc is oxidized in this reaction. So, Zn is a reducing agent.)
Note:
Nitrous acid (HNO2), sulphur dioxide (SO2) and sulphurous acid (H2SO3) are both reducing and oxidising agents.
This is because N and S atoms occur in intermediate oxidation state in these substances.
6. Redox reaction
It is a reaction in which oxidation and reduction occur at the same time.
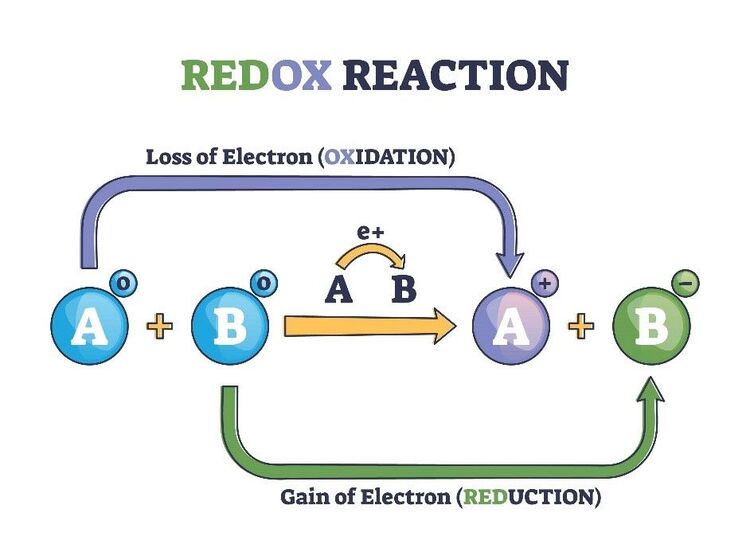
Other examples of Redox reactions are given below:
ZnO + C → Zn + CO
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
7. Oxidation Number
It is the charge found on atom of a molecule or ion. It is also known as oxidation state. An element’s oxidation number can never be higher than number of valence electrons found in it.
8. How to calculate oxidation number?
Oxidation number is calculated with the help of the rules given below.
- An atom’s oxidation number in its free/elemental/uncombined state is zero. For example, oxidation number of H, S and P in H2, S8 and P4 is zero. The oxidation number of O is zero in both O2 and O3.
- Oxidation number of ions made up of only one atom is same to the charge on ion. For example, oxidation numbers of Na+, Mg2+, Fe3+, Cl- and O2- ions are +1, +2, +3, -1 and-2 respectively.
- Oxidation number of all alkali metals (Li, Na, K, Rb, Cs) in their compounds is +1.
- Oxidation number of all alkaline earth metals (Be, Mg, Ca, Sr, Ba) is +2.
- Oxidation number of Aluminium in all its compounds is regarded as +3.
- In most compounds, oxygen has oxidation number of -2.
- In peroxides (H2O2, Na2O2 etc), each oxygen atom has an oxidation number of -1.
- In superoxides (KO2, RbO2), each oxygen atom has an oxidation number of -1/2.
- Oxygen has an oxidation number of +2 and +1 in oxygen difluoride (OF2) and dioxygen difluoride (O2F2) respectively.
- Hydrogen has oxidation number of +1. It has oxidation number of -1 when it bonds with metals in compounds having two elements (LiH, NaH and CaH2).
- The oxidation number of fluorine is -1 in all its compounds. Oxidation number of remaining halogens (Cl, Br and I) is -1 when they are found as halide ions.
- Sum of oxidation number of all atoms in a compound is zero.
- Sum of all oxidation numbers of atoms of the ion is equal to the charge on the ion. For example, in carbonate ion, (CO3)2-, the sum of 3 oxygen atoms and 1 carbon atom must equal -2.
Note:
In carbon dioxide (CO2), carbon occurs in its maximum oxidation state (+4). Further oxidation of carbon is not possible.
Carbon forms compounds in all oxidation states ranging from -4 (in CH4) to +4 (in CO2).
Oxidation state of Nitrogen in various compounds ranges from -3 to +5.
Examples showing how to calculate oxidation number:
1. Oxidation number of Mn in KMnO4
Let us assume that oxidation number of Mn = X. According to previously given rules, Oxidation number of K = +1 and Oxidation number of O = -2.
1+X+ (-2) x 4 = 0
1+X-8 = 0
X = +7
Hence, oxidation number of Mn in KMnO4 is +7.
2. Oxidation number Cr in K2Cr2O7
Let us assume that oxidation number of Cr = X. According to previously given rules, Oxidation number of K = +1 and Oxidation number of O = -2.
1 x 2 + X x 2 + (-2) x 7 = 0
2 + 2X -14 = 0
2X – 12 = 0
X = +6
Hence, oxidation number Cr in K2Cr2O7 is +6.
Summary
Classical concept of oxidation: Oxidation is addition of oxygen or removal of hydrogen, addition of electronegative element/group or removal of electropositive element/group.
Classical concept of reduction: Reduction is addition of hydrogen or removal of oxygen, addition of electropositive element/group or removal of electronegative element/group
Modern concept of oxidation: Oxidation is loss of electrons.
Modern concept of reduction: Reduction is gain of electrons.
Effects of Oxidation in Everyday Life: Corrosion (Rusting of iron, black coating on silver and the green coating on copper), rancidity of fats and oils.
Oxidizing agent (oxidant): undergoes reduction, acceptor of electron, gets reduced, its oxidation number is decreased, examples (O2, O3, H2O2, KMnO4, K2Cr2O7)
Reducing agent (reductant): undergoes oxidation, donor of electrons, gets oxidized, its oxidation number is increased, examples (H2, SO2, CO, H2S, C)
Redox reaction: A reaction in which oxidation and reduction occur simultaneously.
Oxidation Number: Charge found on atom of a molecule or ion. It can never be higher than number of valence electrons found in an element.




 Latest
Latest 



Comments