Chemical effects of Electric Current Class 8 Notes NCERT and MCQs
 23-08-2023
23-08-2023
 17:13 PM IST
17:13 PM IST
 Yadvendra Singh
Yadvendra Singh
The chapter defines poor and goods conductors of electricity. It discusses chemical effects of electric current, electroplating and its applications.
Conductors of electricity
Materials, which allow electric current to pass through them, are good conductors of electricity. Some liquids are good conductors of electricity and some are poor conductors. Poor conductors of electricity do not allow electric current to pass through them easily. Most liquids that conduct electricity are solutions of acids, bases and salts.
Chemical effects of electric current
The passage of an electric current through a conducting liquid causes chemical reactions. The resulting effects are called chemical effects of currents. In 1800, William Nicholsan showed production of oxygen and hydrogen bubbles, if electrodes were immersed in water and current was passed. One of the most common applications of chemical effects of electric current is electroplating.
Electroplating
The process of depositing a layer of any desired metal on another material, by means of electricity, is called electroplating. It is widely used in industry. Some applications of electroplating are given below.
- Chromium plating is done on many objects such as car parts, bath taps, kitchen gas burners, bicycle handlebars, and wheel rims. Chromium has a shiny appearance, does not corrode and resists scratches.
- Electroplating of silver and gold is done on ornaments made up of less expensive metals to give them appearance of silver or gold.
- Tin cans are made by electroplating tin on iron. They are used for storing food as tin is less reactive than iron and food is protected from getting spoilt.
- Zinc coating is deposited on iron on bridges and automobiles to protect them from corrosion and rusting.
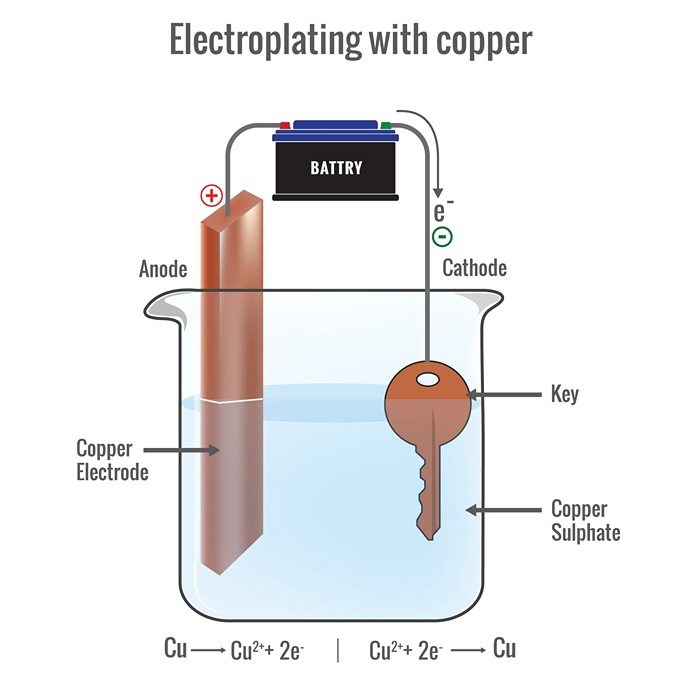
Fig.1: Electroplating with copper
MCQs based on NCERT Class 8 Science Chapter 14: Chemical effects of electric current
1. Which of the following metals is deposited on car parts, bath taps, kitchen gas burners, bicycle handlebars, and wheel rims to give them shiny appearance and prevent corrosion?
a. Chromium
b. Silver
c. Tin
d. Zinc
And a
Explanation:
Chromium is deposited on car parts, bath taps, kitchen gas burners, bicycle handlebars, and wheel rims to give them shiny appearance and prevent corrosion. Electroplating of silver and gold is done on ornaments made up of less expensive metals to give them appearance of silver or gold. Tin cans are made by electroplating tin on iron. Zinc coating is deposited on iron on bridges and automobiles to protect them from corrosion and rusting.
2. Which of the following metal is coated on iron on bridges and automobiles to protect them from corrosion and rusting?
a. Silver
b. Tin
c. Zinc
d. Chromium
Ans. b
Explanation:
Tin is coated on iron on bridges and automobiles to protect them from corrosion and rusting. Silver and gold is coated on ornaments made up of less expensive metals to give them appearance of silver or gold. Tin cans are made by electroplating tin on iron. Chromium is deposited on car parts, bath taps, kitchen gas burners, bicycle handlebars, and wheel rims to give them shiny appearance and prevent corrosion.
Frequently Asked Questions (FAQs) about Chemical Effects of Electric Current
What is electroplating?
What are good conductors of electricity?
Share Blog
 Latest
Latest 
Comments